Bio-Analytical SPE Method: Semaglutide
Quantitative Estimation from Human K2 EDTA Plasma Samples
Method Overview
Quantitative Estimation of Semaglutide from Human K2 EDTA plasma samples
Semaglutide is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management, developed by Novo Nordisk in 2012. Semaglutide is a glucagon-like peptide-1 receptor agonist, meaning that it mimics the action of the human incretin glucagon-like peptide-1 (GLP-1), thereby increasing insulin secretion and blood sugar disposal and improving glycemic control.
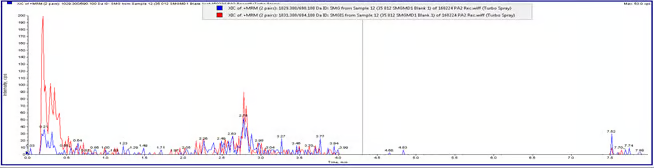
Chromatogram 1: Blank Plasma Sample
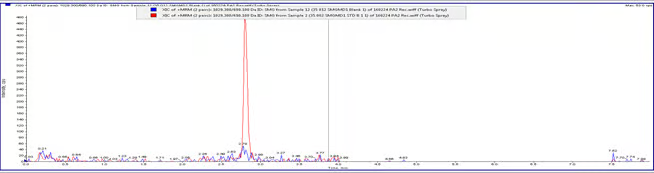
Chromatogram 2: LLOQ (0.5 ng/ml) overlay on Blank Plasma sample
Method Details & Results
Results Summary
| Experiments | Results |
|---|---|
| S/N | >20:1 for LLOQ 0.5ng/ml |
| Selectivity | Interference <20% of LLOQ in Normal, haemolytic and lipemic plasma |
| Recovery | 73, 75 and 73% at LQC, MQC and HQC levels |
| Precision | -1.02 to 6.27% for LLOQ, LQC, MQC and HQC levels |
| Accuracy | 3.09 to 5.88% for LLOQ, LQC, MQC and HQC levels |
| Stability | All matrix stabilities are within specifications |
| Long term stability | Samples are stable at -20°C for 105 days |